Advanced thermoelectric materials could be used to develop vehicle exhaust systems that convert exhaust heat into electricity, concentrate solar energy for power generation and recover waste heat from industrial processes.
June 10, 2011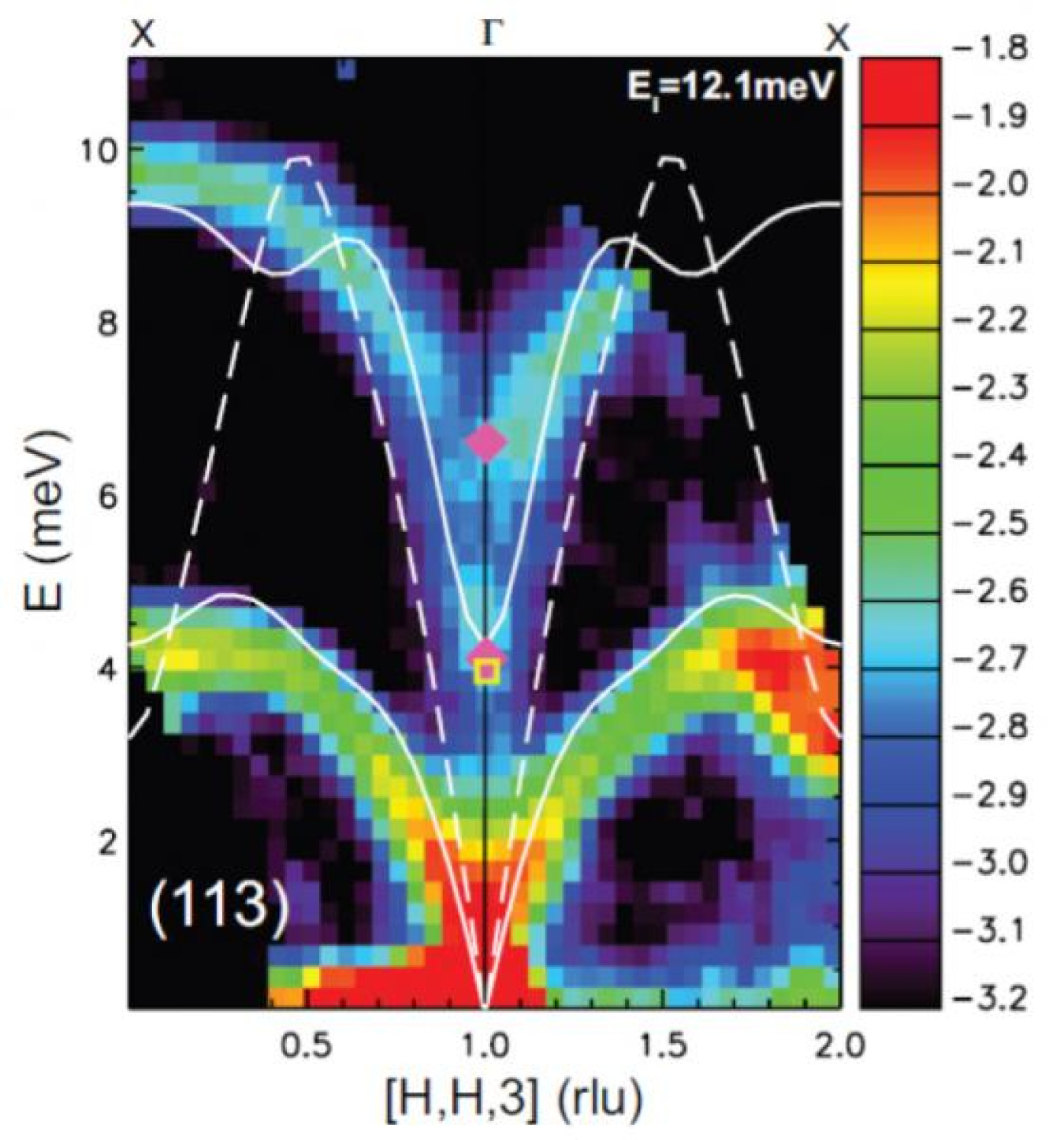
Data image on lead telluride thermal conductivity | Photo Courtesy of Oak Ridge National Laboratory
With the ability to control conductivity in thermoelectrics comes great power. Through this, less energy is dispersed and more heat can be directed to power generation. For example, advanced thermoelectric materials could be used to develop vehicle exhaust systems that convert exhaust heat into electricity, concentrate solar energy for power generation and recover waste heat from industrial processes. Controlled thermal conductivity can be applied to deep-space probes that use heat from decaying radioisotopes to generate power.
Through funding from one of the Department’s Energy Frontier Research Centers, Oak Ridge National Laboratory scientists are conducting neutron analysis of the atomic dynamics behind thermal conductivity. Researchers performed experiments at the laboratory’s two neutron facilities – Spallation Neutron Source and the High Flux Isotope Reactor – to learn why lead telluride, a material with a similar molecular structure to table salt, has very low thermal conductivity.
The Oak Ridge scientists discovered that an unusual coupling of microscopic vibrational modes, called phonons, is responsible for disrupting structures normally used to transport thermal energy, canceling the material’s ability to conduct heat.
Oak Ridge researcher Olivier Delaire noted, “The microscopic origin of the low thermal conductivity is not well understood. Once we do understand it better we can design materials that perform better at converting heat to electricity.”
Check out more on this neutron analysis of thermal conductivity here.
At Ames Laboratory, scientists have identified a key ingredient in bone’s nanostructure, which may help treat and prevent bone diseases such as osteoporosis and develop new light-weight, high-strength materials for innovative technologies.
Ames scientist and Iowa State University chemistry professor Klaus Schmidt-Rohr explained, “The organic, collagen matrix is what makes bones tough, while the inorganic apatite nanocrystals provide the stiffness. And the small thickness – about 3 nanometers – of these nanocrystals appears to provide favorable mechanical properties, primarily in prevention of crack propagation.”
While bone structure has been studied extensively, how these apatite nanocrystals form and what prevents them from growing thicker was a mystery. Through a bit of luck and creative hypothesizing, Schmidt-Rohr and his team found that the higher the concentration of citrate, the thinner the apatite nanocrystals in bone. At higher concentrations, the nanocrystals that formed were thinner and therefore are likely more resistant to crack propagation.
Read more on why citrate is key to bone’s nanostructure here.


