-- This project is inactive --
Southern Research Institute (SRI), through the Concentrating Solar Power: Efficiently Leveraging Equilibrium Mechanisms for Engineering New Thermochemical Storage (CSP: ELEMENTS) funding program, is developing thermochemical energy storage (TCES) systems for CSP based on endothermic-exothermic gas-solid reaction cycles at temperatures >650°C. The project develops and demonstrates regenerative carbonate and silicate sorbent-based process in a simulated TCES system at bench scale. Upon completion, the project will advance the proposed TCES system from TRL-2 to TRL-4 by demonstrating the system's key advantages, its high exergetic and energetic efficiencies, and its potential to meet a cost target of $15/kWhth.
Approach
Tasks include the investigation of sorbent preparation methods, TGA/DSC cyclic testing for simultaneous measurement of heat flow and reaction extent, optimization of preparation with appropriate promoters, computational design of an advanced heat-exchange reactor system, evaluation of the optimized pellets in a simulated bench-scale TCES system over multiple cycles, and Aspen modeling and costing of commercial system embodiment.
Innovation
The proposed TCES system is based on the ability of advanced sorbent materials to repeatedly undergo endothermic-exothermic gas-solid reaction cycles with a heat of reaction of 130-180 kJ/mol at 650-850°C. The proposed sorbent-based TCES system appears to have significant advantages over other competing systems. These advantages include high selectively, rapid kinetics, negligible side reactions, high cyclic stability, and low cost. Through experimental and modeling studies, the project seeks to advance the proposed TCES system from TRL-2 to TRL-4 by demonstrating the system’s key advantages, its high exergetic and energetic efficiencies, and its potential to meet a cost target of $15/kWhth.
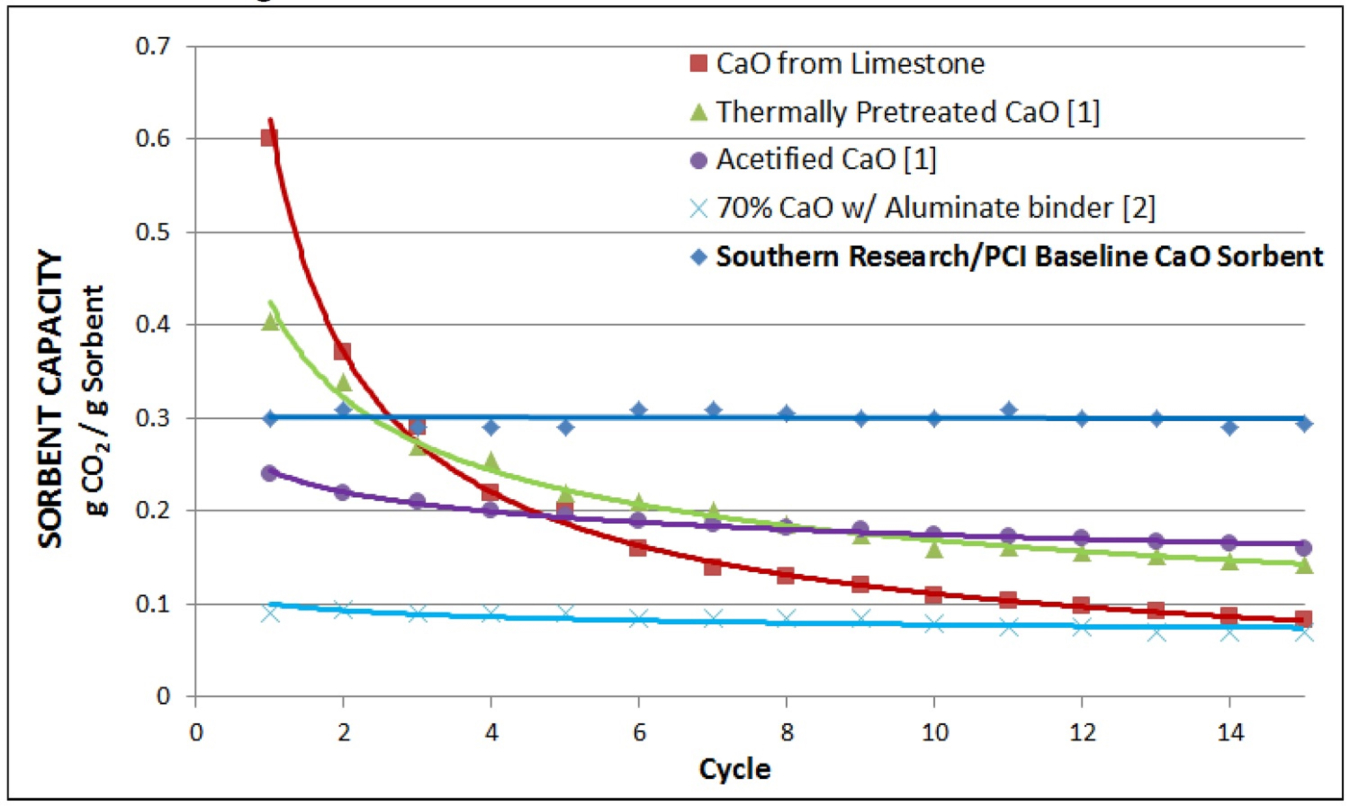
The modified calcium carbonate sorbents under investigation by SRI for solar TCES is subjected to hundreds of cycles in simultaneous TGA/DSC equipment to measure both mass and heat change as the material undergoes repeated high temperature carbonation/decarbonation reactions. Initial experimental results, shown by the blue diamonds in the graph to the left, suggest that the proposed system has greater stability than competing formulations.
Learn about other concentrating solar power research.
