This text version of the fuel cell animation demonstrates how a fuel cell uses hydrogen to produce electricity, with only water and heat as byproducts.
Introduction
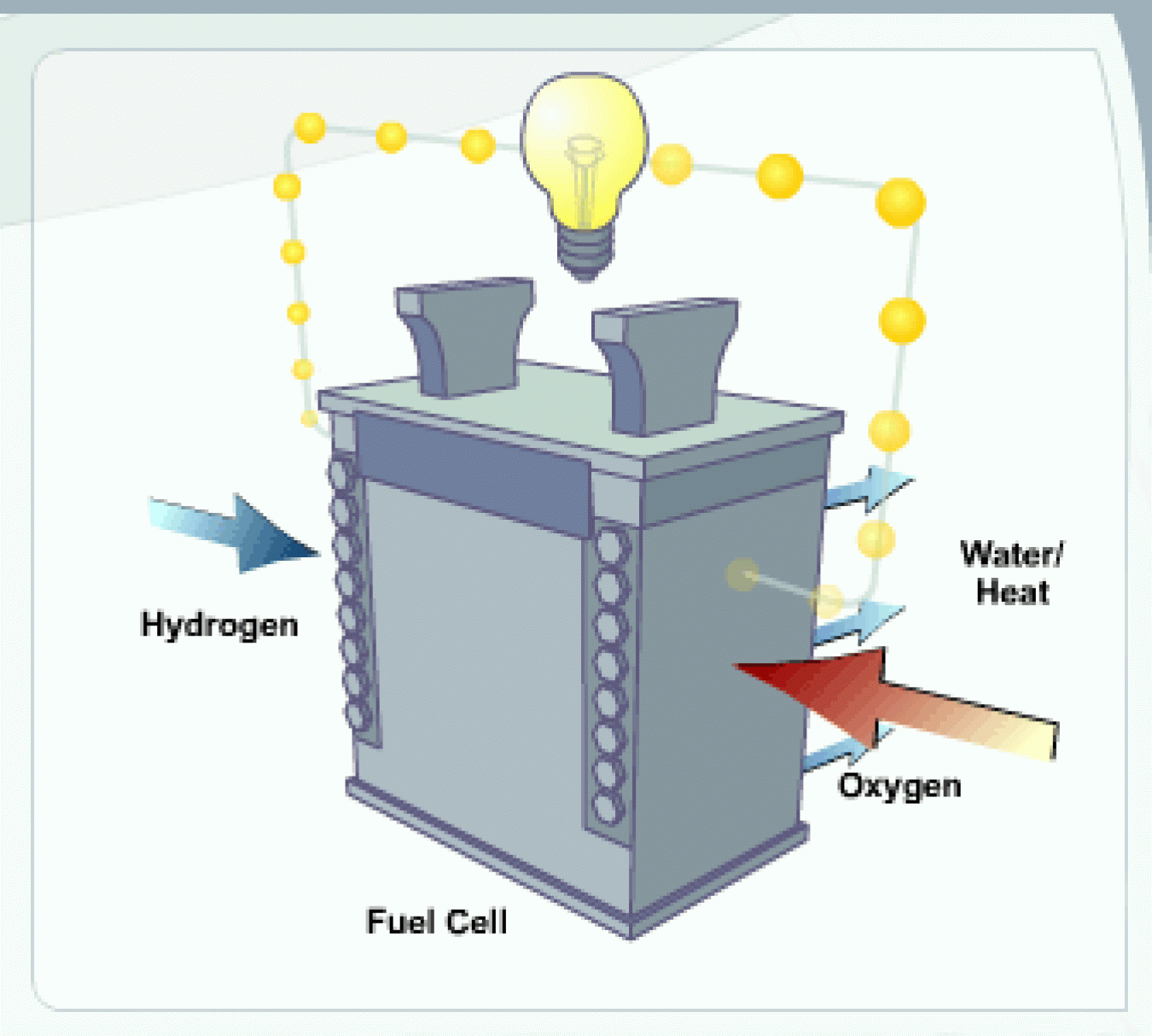
A fuel cell is a device that uses hydrogen (or hydrogen-rich fuel) and oxygen to create electricity. Fuel cells are more energy efficient than combustion engines and the hydrogen used to power them can come from a variety of sources. If pure hydrogen is used as a fuel, fuel cells emit only heat and water, eliminating concerns about air pollutants or greenhouse gases.
Fuel Cell Components
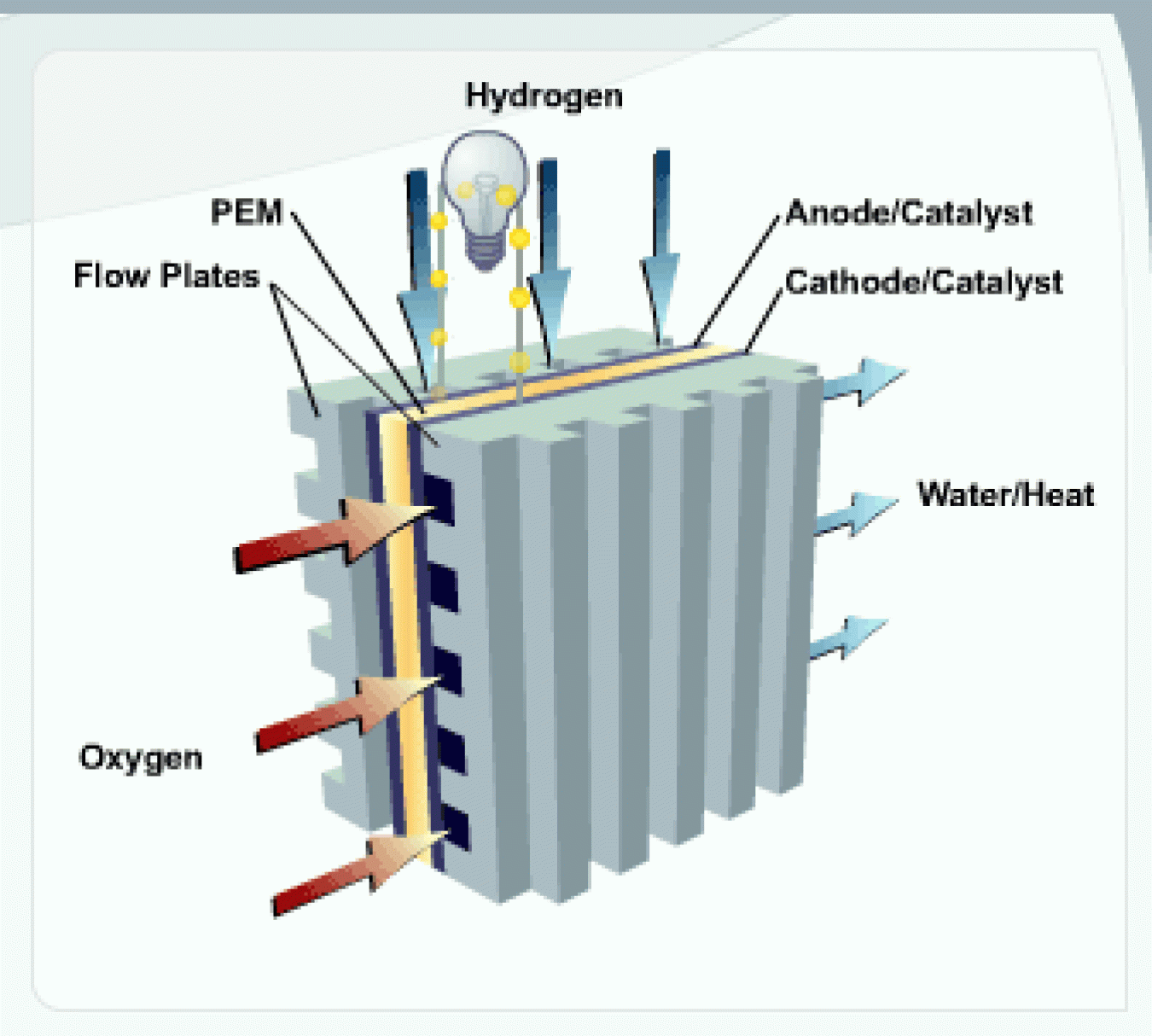
One of the more common types of fuel cell is the polymer electrolyte membrane (PEM) fuel cell. The PEM fuel cell consists of an electrolyte membrane sandwiched between an anode (negative electrode) and a cathode (positive electrode).
PEM
The PEM is a thin, solid, organic compound, typically the consistency of plastic wrap and about as thick as 2 to 7 sheets of paper. This membrane functions as an electrolyte: a substance that conducts charged ions (in this case protons), but does not conduct electrons. This allows the solution to conduct electricity. This membrane must be kept moist to conduct particles through it.
Anode
The anode is the electrode at which oxidation (loss of electrons) takes place. In a fuel cell, the anode is electrically negative. The anode is composed of platinum particles uniformly supported on carbon particles. The platinum acts as a catalyst, increasing the rate of the oxidation process. The anode is porous so that hydrogen can pass through it.
Cathode
The cathode is the electrode at which reduction (gaining of electrons) takes place. In a fuel cell, the cathode is electrically positive. The cathode is composed of platinum particles uniformly supported on carbon particles. The platinum acts as a catalyst, increasing the rate of the reduction process. The cathode is porous so that oxygen can pass through it.
Flow Plates
Flow plates perform several important functions:
- They channel hydrogen and oxygen to the electrodes
- They channel water and heat away from the fuel cell
- They conduct electrons from the anode to the electrical circuit and from the circuit back to the cathode.
The Chemical Process
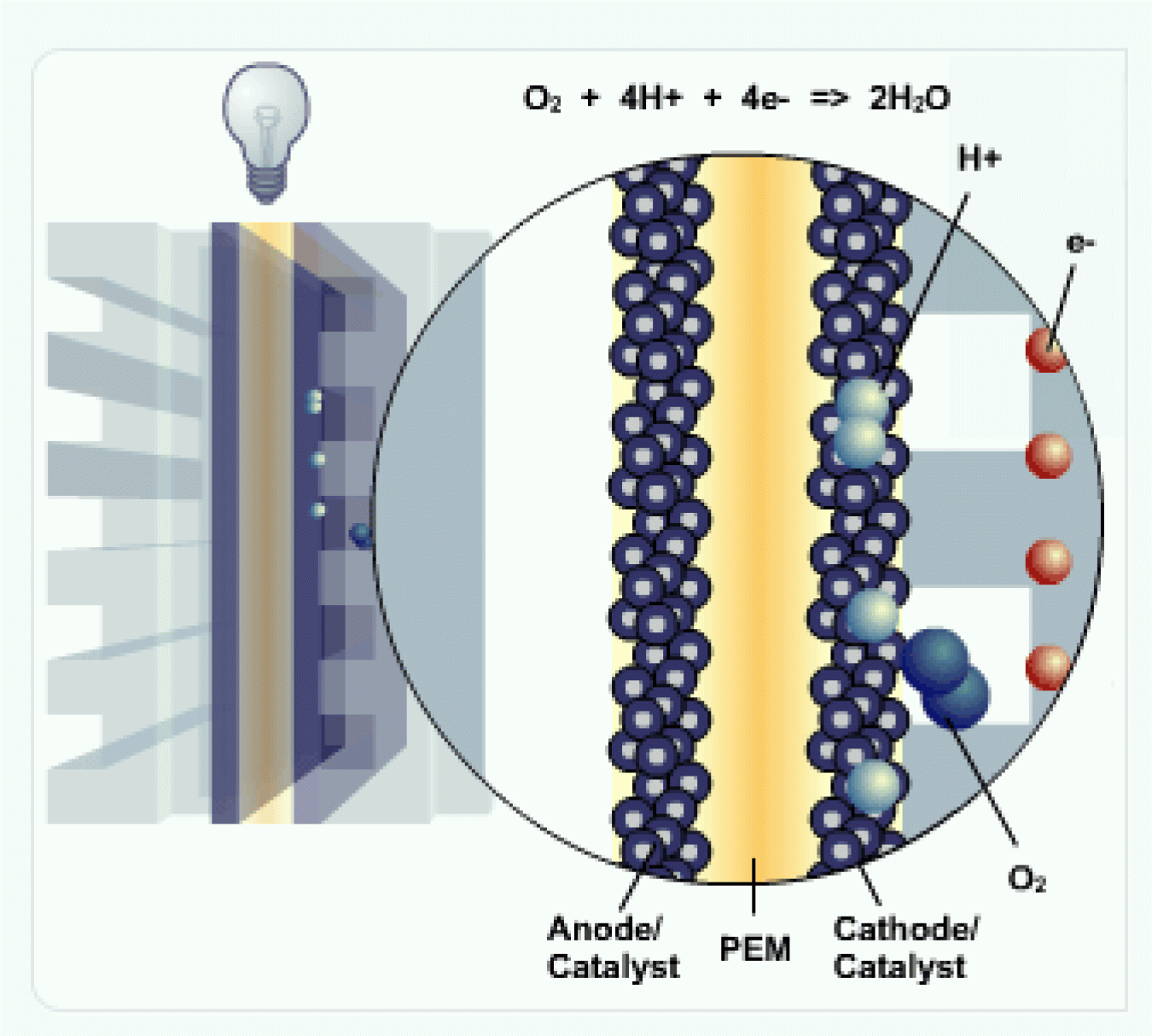
H2 and Anode
Hydrogen fuel (H2) is channeled to the anode, where the catalyst separates the hydrogen's negatively charged electrons from the positively charged protons.
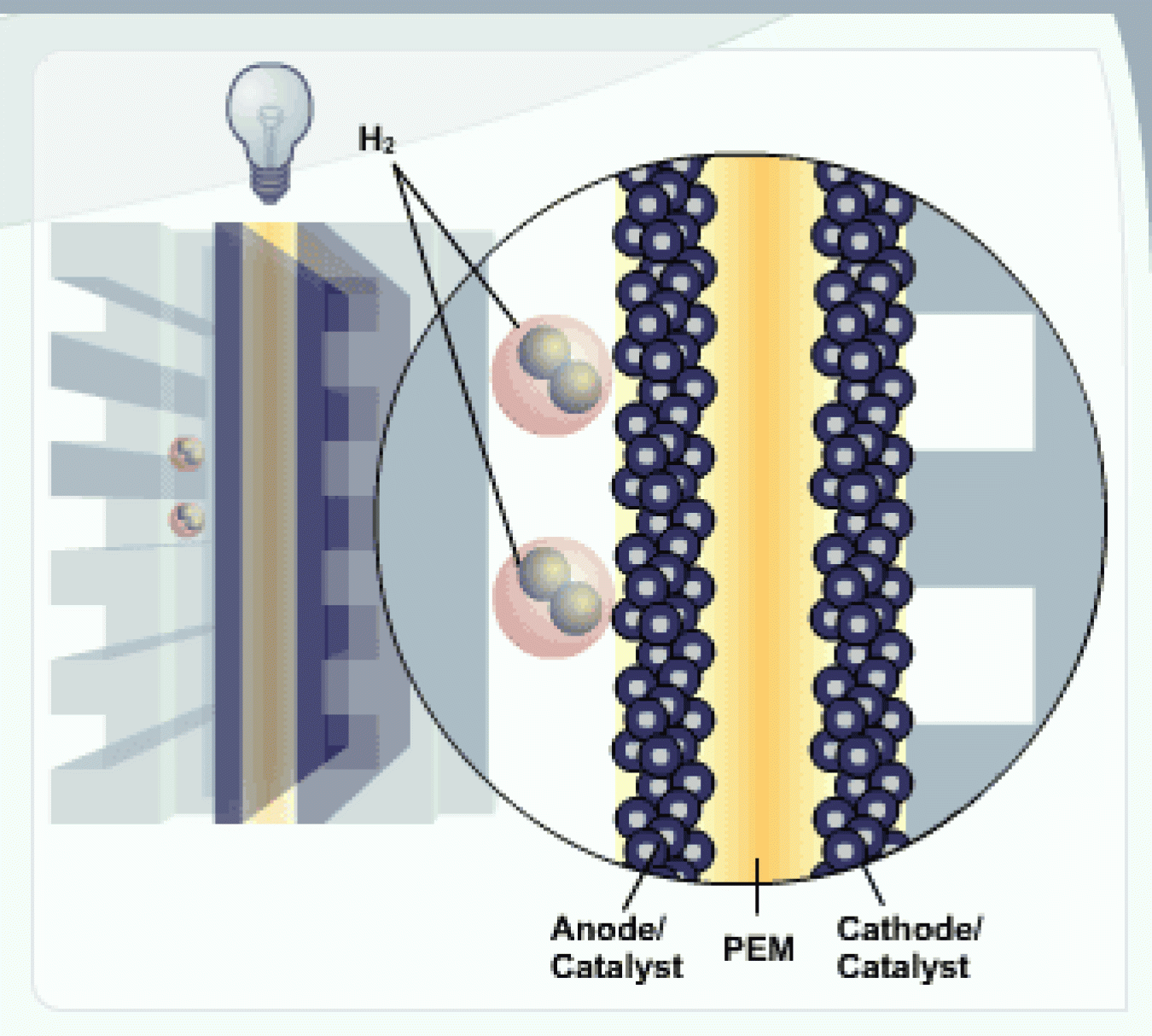
PEM
The membrane allows the positively charged protons to pass through to the cathode, but not the negatively charged electrons.
The negatively charged electrons must flow around the membrane through an external circuit. This flow of electrons forms an electrical current.
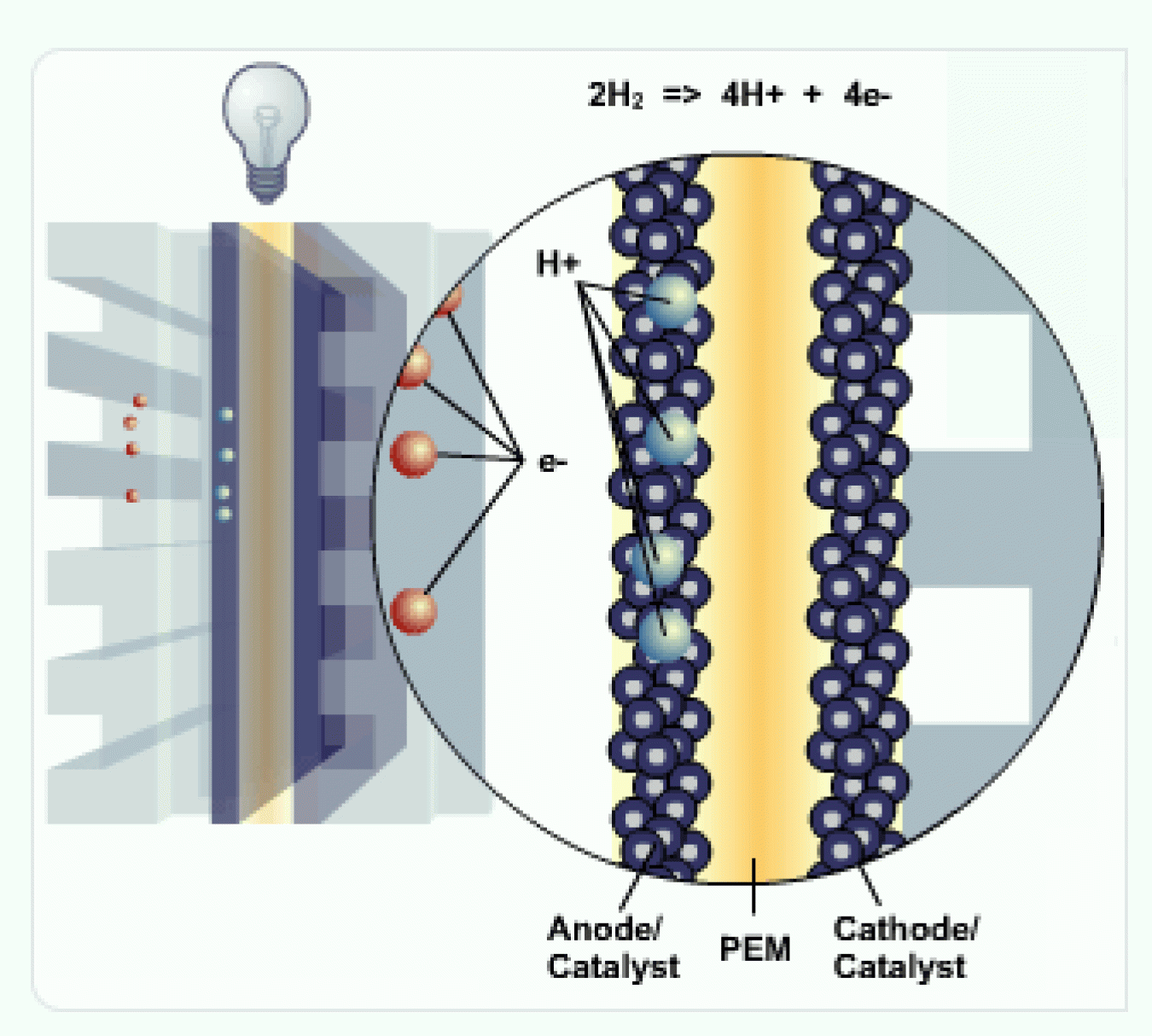
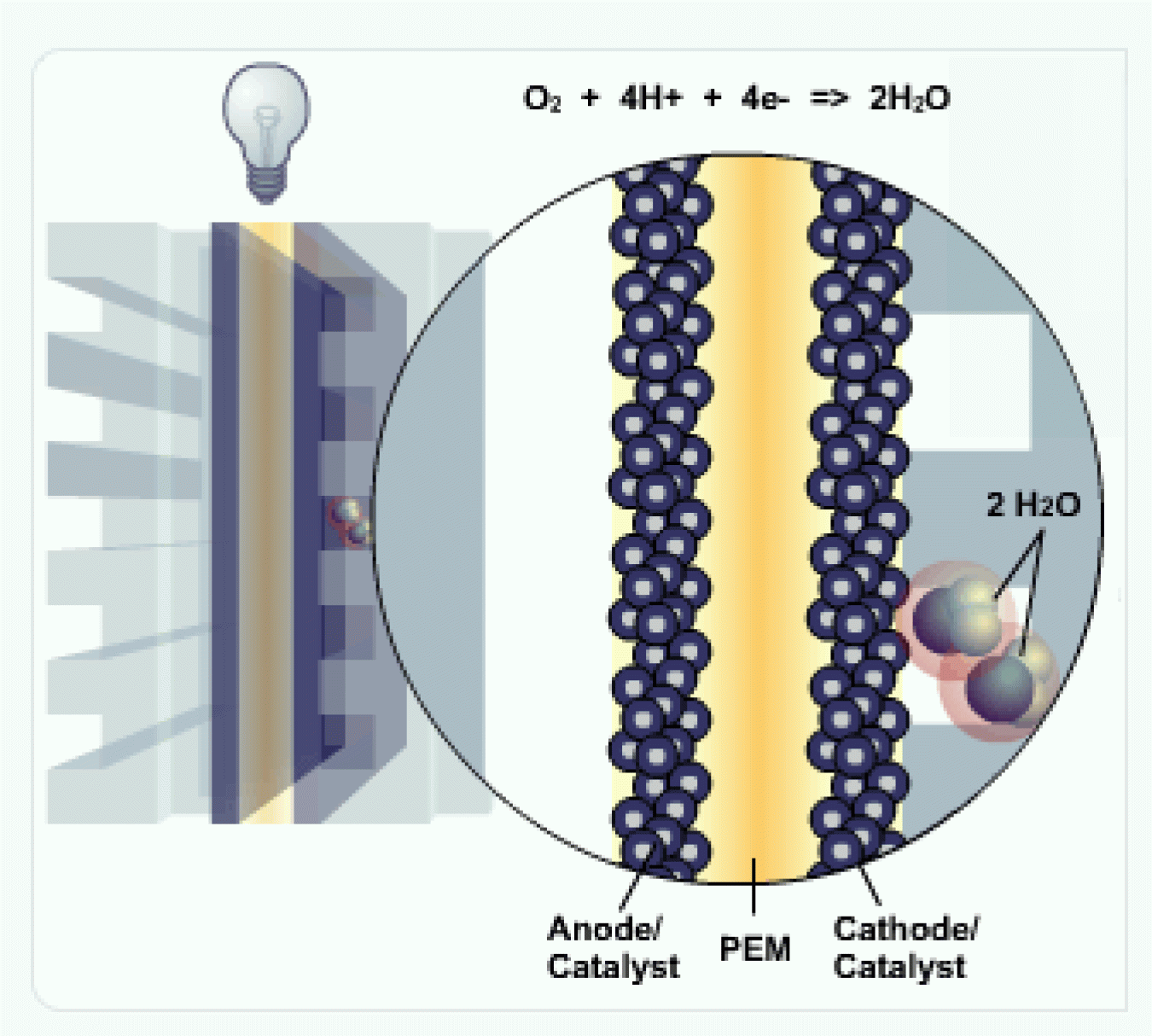
Cathode
At the cathode, the negatively charged electrons and positively charged hydrogen ions (protons) combine with oxygen to form water (H2O) and heat.
Fuel Cell Stack
Fuel Cell
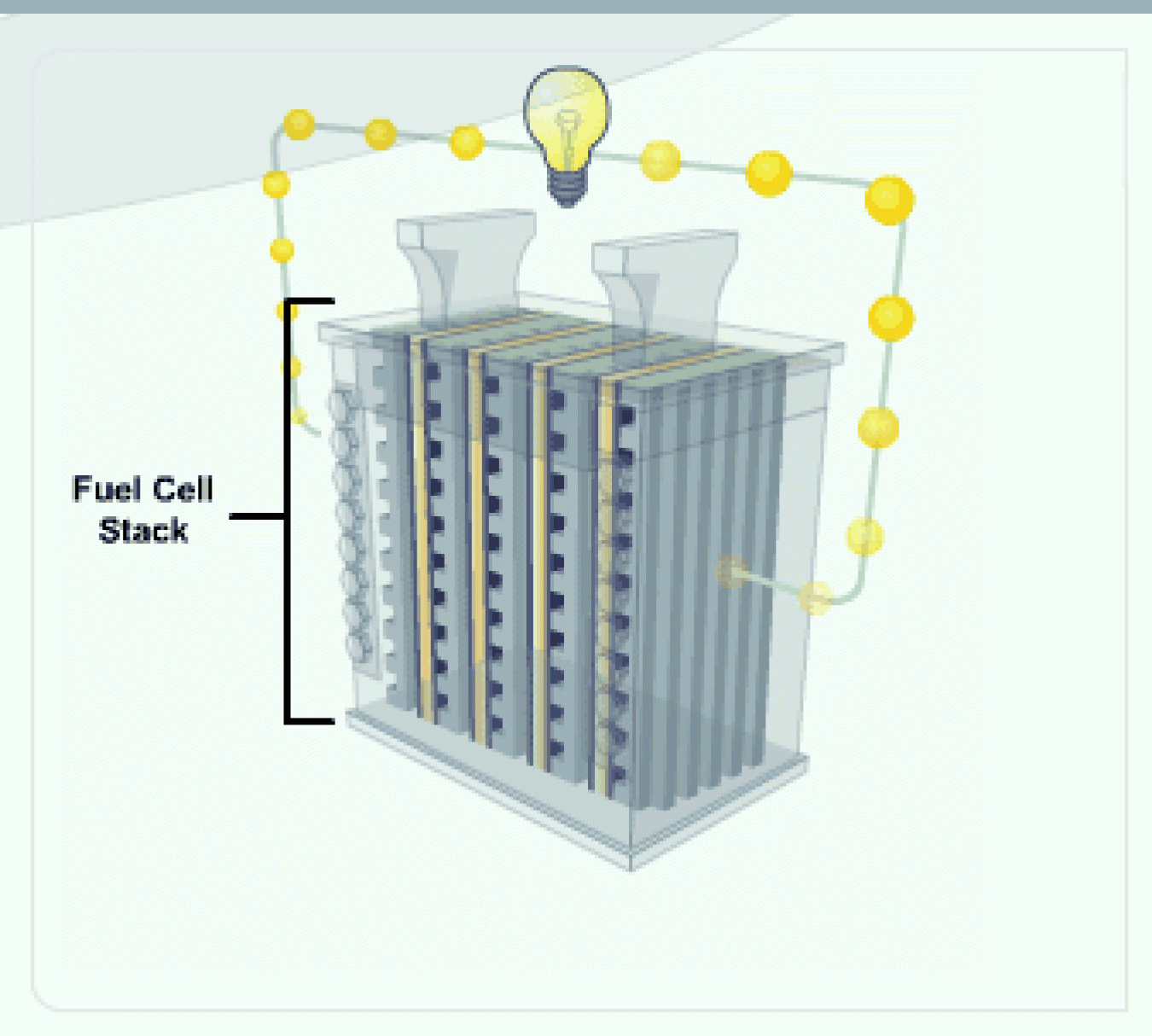
The amount of power produced by a fuel cell depends on several factors, including fuel cell type, cell size, temperature at which it operates, and pressure at which the gases are supplied to the cell. A single fuel cell produces less than 1.16 volts—barely enough electricity for even the smallest applications.
Fuel Cell Stack
To increase the amount of electricity generated, individual fuel cells are combined in series, into a fuel cell "stack." A typical fuel cell stack may consist of hundreds of fuel cells.
Fuel cells are a flexible technology and have a broad range of applications.
Transportation
Fuel cells can be used to provide propulsion or auxiliary power for transportation applications including cars, trucks, buses, trains, ships, and submarines. They have been used to provide auxiliary power on spacecraft for decades.
Stationary Power
Stationary fuel cell units can be used for backup power, power for remote locations, stand-alone power plants for towns and cities, distributed generation for buildings, and co-generation (in which excess thermal energy from electricity generation is used for heat).
Portable Power
Fuel cells can be used to power a variety of portable devices, from handheld electronics like cell phones and radios, to larger equipment such as portable generators. They can be used for almost any application typically powered by batteries but can last up to three times longer before refueling.
